On December 10-11, the WHO Technical Expert Group (TEG) on Drug Efficacy and Response met in Geneva to make recommendations on the appropriate response to the spread of artemisinin resistance, and to look at the effects of future malaria control strategies such as seasonal malaria chemoprophylaxis, triple combination therapies, and the deployment of multiple first-line therapies (MFT).
The results of our December publication in Lancet Global Health were presented at this meeting, and the TEG acknowledged that MFT was likely to delay drug-resistance emergence and that a WHO recommendation should be considered. One of the major concerns about MFT strategies is that simultaneous deployment of multiple drugs may allow for the early emergence of a multi-drug resistant genotype, via genetic recombination between two parasites with different types of drug resistance. Our published model included a sub-model of genetic recombination when a mosquito took up two genetically different gametocytes from an individual with a multi-clonal infection, but we did not allow for interrupted feeding by mosquitoes.
For the December WHO meeting, we updated our individual-based model to include interrupted feeds by mosquitoes. This mechanism allows for the possibility that a mosquito takes up two different drug-resistant parasites from two different individuals, that these parasites recombine inside the mosquito to form a new multi-drug-resistant genotype, and that this multi-drug-resistant genotype is then transmitted to a new individual. This is a real possibility under and MFT strategy, as an MFT strategy may generate multiple types of single-drug-resistant parasites that will then be able to be combined into a multi-drug-resistant genotype if they are present in the mosquito at the same time.
The results below show the strategy comparisons when interrupted feeding is included in the model
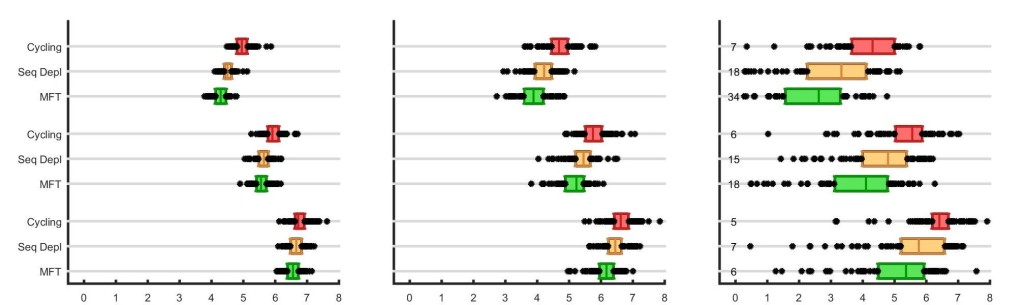
The x-axis shows the number of treatment failures (NTF) over a 20-year simulation, and the boxes show interquartile ranges from 100 simulation runs. As in our previous post, the treatment coverage increases from 50% to 60% to 70% in the three panels, and the numbers in the rightmost panel show the number of times malaria was eliminated in 100 simulations.
Why does multiple first-line therapies still outperform the two cycling strategies when recombination is included? The reason is that recombination works in both directions. It brings together single-resistant genotypes to form double-resistants, and it breaks apart double-resistants to form single-resistants. In fact, the recombination breakdown process may be the faster one, as an emerging double-resistant strain has a very high chance to be paired up with a fully-sensitive strain in the early stages of a drug-resistance epidemic; in this scenario, the double-resistant would have a 50% chance of being broken down by recombination.
To date, MFT as a public health policy for malaria has been evaluated by a small number modeling analyses:
The WHO Technical Expert Group on Drug Efficacy and Response recommended that more modeling groups generate evidence — from independent mathematical models — supporting or contradicting the benefits of MFT relative to other drug deployment strategies. The full text of their recommendation is reproduced below.
In areas where there is no established MDR [multidrug resistance], simultaneous deployment of multiple effective ACT first-line treatments (MFLT) is unlikely to hasten, and may actually delay, the emergence of drug resistance, according to modelling studies. Therefore:
-
- countries that presently have multiple approved ACT first-line treatments should continue to use them; and
- countries that rely on a single ACT first-line treatment are encouraged to add additional effective ACT treatments to the national treatment guidelines, both to potentially delay the onset of resistance and to be better prepared to respond to failure (or stock-outs) of the current first-line treatment.
Because modelling is the only means of evaluating the impact of MFLT on delaying resistance, the TEG recommends that the Malaria Modelling Consortium be asked to develop these modelling approaches. Implementation issues should also be considered. The DER TEG is ready to examine outputs from the Malaria Modelling Consortium and any supporting clinical data.
The minutes from the December 2015 WHO TEG can be viewed here.